You cant merely draw a Lewis dot construction for a metallic it’s important to know the metallic. There are 2 lone pairs of electrons on every O atom and As has 1 lone pair of electrons on every atom.
 Nancy Nguyen Chemistry Of Supplies 2013
Nancy Nguyen Chemistry Of Supplies 2013
Since nickel is a transition factor it’s important to manually write out its electron configuration and determine what number of electrons the l.
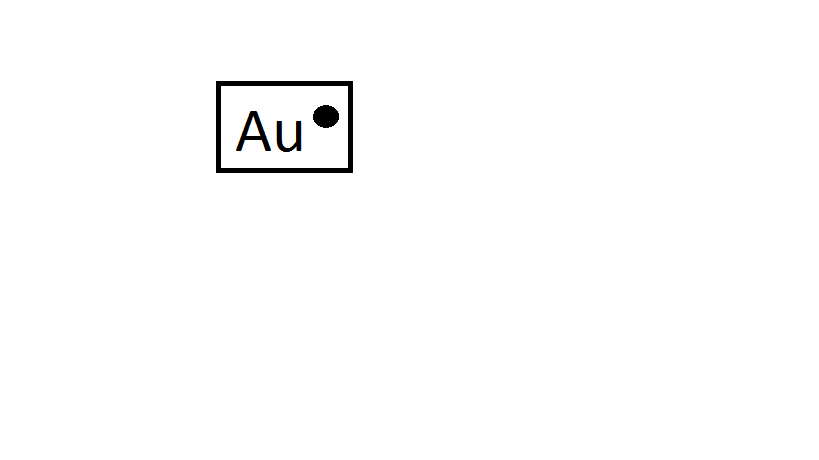
Gold lewis dot construction. Predicted knowledge is generated utilizing the ACDLabs Percepta Platform – PhysChem Module. These valence electrons are negatively charged and are drawn to the positively charged nucleus made up of neutrons and protons. The Lewis construction would appear like.
When this example happens the molecules Lewis construction is alleged to be a resonance construction and the molecule exists as a resonance hybrid. Lewis outlined a base as an electron pair donor and an acid as an electron pair acceptor. Is it essential for the primary dot round an atomic image to go on a selected aspect of the atomic image.
Golds Identify in Different Languages. Take for instance nitrate ion N O 3. Discover the full valence electrons for the molecule.
Oxygen Group VI has 6 valence electrons. Lewis dot construction is the classical bonding mannequin by which solely valence electrons of the atoms are used. As a substitute of performing like an entitled egomaniac who thinks hes the neatest man within the room like the opposite man did In poor health present an precise reply.
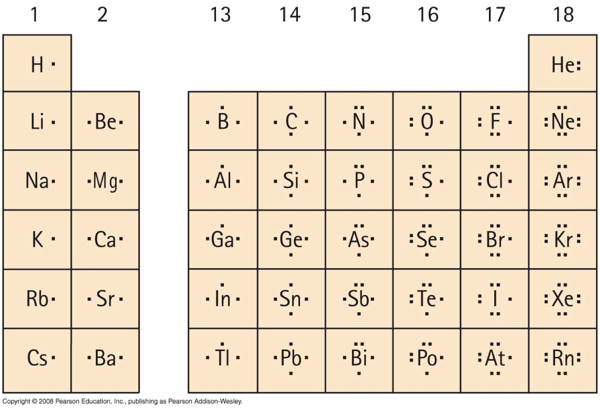
Index Chemical ideas Chemistry of the Components Periodic Desk. Electron Distributions Into Shells for the First Three Durations. Many components don’t observe the octet rule.
Lewis construction is essential in chemistry as a result of they’re utilized in many essential ideas of basic chemistry akin to chemical bonding resonance valence shell electron pair repulsion principle prediction of. H 2 S NCl 3 OH-Put the least electronegative atom within the middle. Reply 1 of two.
There are 2 lone pairs of electrons on every O atom and As has 1 lone pair of electrons on every atom. Draw Lewis Dot Construction. Lewis dot buildings are generally known as electron dot buildings or Lewis buildings.
Lewis dot buildings mirror the digital buildings of the weather together with how the electrons are paired. A double bond is represented by two pairs of dots. Nitrogen Group V has 5 valence electrons.
CH 4 NH 3 I 2. Put two electrons between atoms to type a chemical bond. 254 C LabNetwork previous LN00202325.
Cu If you happen to had a pair of coppers you’d write CuCu Gold is actually the identical. The Lewis Dot Construction is a visible which represents the outermost shell of electrons also called valence electrons and attainable covalent bonds inside an atom or molecule. HyperPhysics Quantum Physics.
H all the time goes outdoors. Exceptions to the Octet Rule. Gold lewis dot construction.
Lewis-Dot Diagrams Lewis Dot Diagrams are a strategy to signify the valence electrons in an atom. Components image represents the nucleus and inner-level electrons Dots signify the valence electrons 53 Electron Configuration. A number of the exceptions about octet rule are given beneath.
A chemical factor is recognized by the variety of protons in its nucleus and it should gather an. If the theoretical calculations are performed rigorously we. Lewis-Dot Diagrams Dots are positioned one after the other on the 4 sides of the image then paired till all valence electrons are used Most of.
Overview of Gold. Full octets on outdoors atoms. What’s the electron dot diagram for carbon.
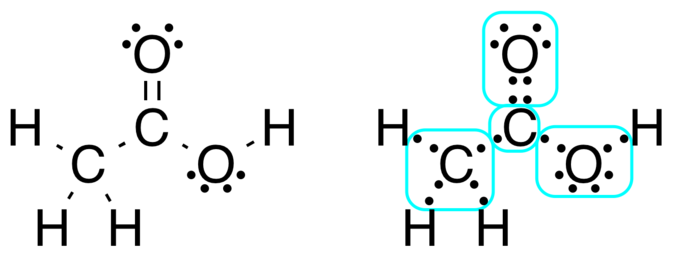
Now we construct Lewis buildings by elaborating from impartial atoms and naturally we now have to account for the cost on the atom or radical ion. Molecular construction by which the valency electrons are proven as dots so positioned between the bonded atoms that one pair of dots represents two electrons or one covalent single bond eg. And we throw in one other electron in order that we now have 5 3 6 1 24 valence.
Electron Configuration into Shells. An electron or molecule which incorporates unpaired electrons in its outermost shell or valence shell is taken into account as free radical. These electrons are much less steady and don’t obey the.
We should always confirm the usefulness of our easy predictions with molecular orbital principle. Steps for Writing Lewis Buildings. These theories which embrace Lewis buildings VSEPR and hybridization are easy fashions that assist predict chemical properties.
Nevertheless Lewis dot buildings and hybridization are approximations which will or might not match actuality. Lewis buildings are a helpful strategy to. NOCl CF 2 Cl 2 HCN.
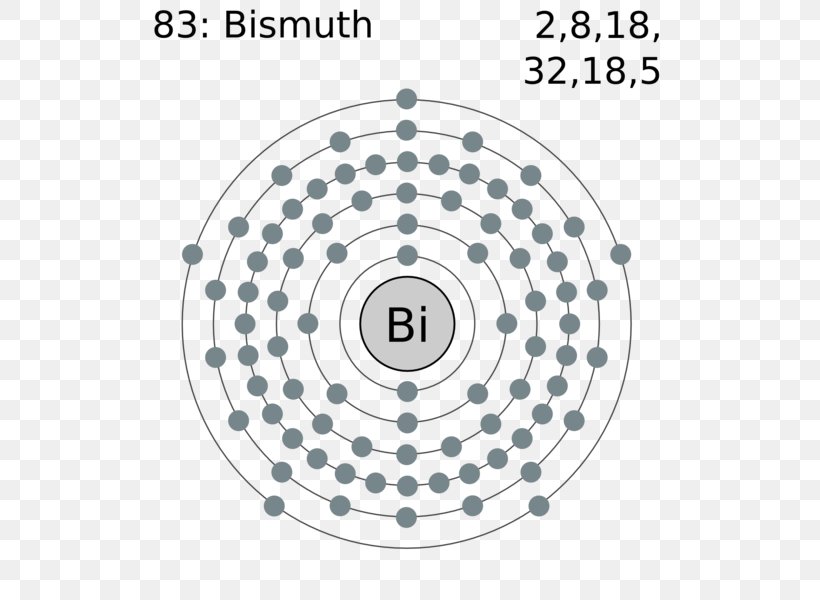
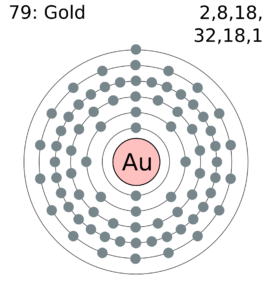
Within the case of copper the electron from the s shell is moved to fill the d shell so there is just one electron within the s shell. Atomic Construction of Gold. Lewis Dot Diagrams of Chosen Components.
One would merely write. It’s proven beneath with the assistance of Lewis dot construction. Lewis Electron Dot Construction Of Gold DIAGRAM Electron Dot Diagram Gold FULL Model HD Introduction to Lewis Buildings for Covalent Molecules Exceptions to the Octet Rule Lewis Electron Dot Buildings CK 12 Basis.
 Lewis Dot Symbols And Lewis Buildings Boundless Chemistry
Lewis Dot Symbols And Lewis Buildings Boundless Chemistry
 Au Electron Configuration Gold Youtube
Au Electron Configuration Gold Youtube
Gold Iii Chloride Aucl3 Chemspider
 Gold Nanoparticles 20 Nm Diameter Od 1 Stabilized Suspension In Citrate Buffer Au Np
Gold Nanoparticles 20 Nm Diameter Od 1 Stabilized Suspension In Citrate Buffer Au Np
 Lewis Electron Dot Buildings Detailed Rationalization With Examples Movies
Lewis Electron Dot Buildings Detailed Rationalization With Examples Movies
 Periodic Community Licensed For Non Business Use Solely Gold
Periodic Community Licensed For Non Business Use Solely Gold
 Lewis Construction Gold Atom Diagram Chemistry Png 549x600px Lewis Construction Space Atom Bohr Mannequin Model Obtain
Lewis Construction Gold Atom Diagram Chemistry Png 549x600px Lewis Construction Space Atom Bohr Mannequin Model Obtain
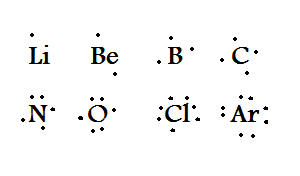 Yr 11 Misadventures Electron Dot Diagrams
Yr 11 Misadventures Electron Dot Diagrams
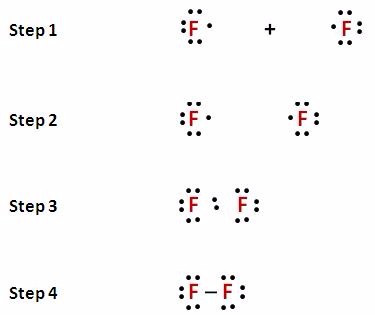
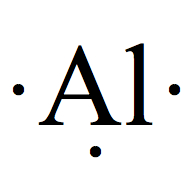 Chapter 7 Covalent Bonds And Molecular Construction
Chapter 7 Covalent Bonds And Molecular Construction
 Lewis Dot Diagram For A number of Molecular Fashions A E And For Bulk Au 2 Obtain Scientific Diagram
Lewis Dot Diagram For A number of Molecular Fashions A E And For Bulk Au 2 Obtain Scientific Diagram
 Gold Valence Electrons Gold Valency Au With Dot Diagram
Gold Valence Electrons Gold Valency Au With Dot Diagram
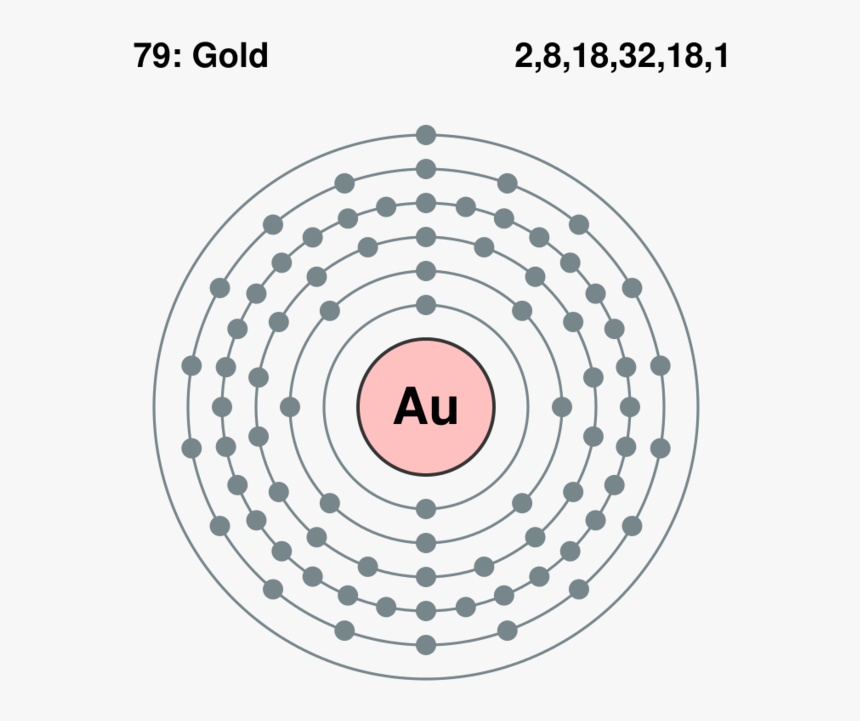 Gold Lewis Dot Construction Hd Png Obtain Kindpng
Gold Lewis Dot Construction Hd Png Obtain Kindpng
 Lewis Construction Of Aucl Gold I Chloride Youtube
Lewis Construction Of Aucl Gold I Chloride Youtube
 Lewis Dot Symbols And Lewis Buildings Boundless Chemistry
Lewis Dot Symbols And Lewis Buildings Boundless Chemistry